| US 5,856,336請求項1 |
US 6,465,477請求項1 |
|
1. A compound of the formula,
一種化學式A之化合物
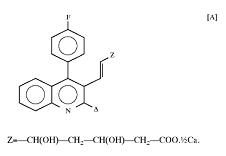
|
1. A pharmaceutical composition comprising (E)-3,5-dihydroxy-7-[4'-4"-flurophenyl-2'-cyclopropyl-quinolin-3'-yl]-6-heptenoic acid, or its salt or ester, and a pharmaceutically acceptable carrier, of which an aqueous solution or dispersion of the pharmaceutical composition has pH of from 6.8 to 7.8.
一種醫藥組合物,其包含化學式(E)-3,5-dihydroxy-7-[4'-4"-flurophenyl-2'-cyclopropyl-quinolin-3'-yl]-6-heptenoic之酸、或其鹽類或內脂,以及醫藥上可接受之載體,其中醫藥組合物之溶液或分散液具備6.8至7.8的酸鹼值 |
| 請求項 2 |
|
|
2. A method for reducing hyperlipidemia, hyperlipoproteinemia or atherosclerosis, which comprises administering an effective amount of the compound of formula A as defined in claim 1.
一種減緩高脂血症、高脂蛋白血症或動脈粥狀硬化之方法,其包括投藥有效劑量之如請求項1所定義的通式A化合物
|
| (本專利並無圖式) |
| US 8,557,993請求項1 |
1. A crystalline polymorph A, B, C, D, E, F, or the amorphous form, of (3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenye)quinolin-3-yl]-3,5-dihydroxy-6(E)-heptenoic acid hemicalcium salt wherein
一種化學式為(3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenye)quinolin-3-yl]-3,5-dihydroxy-6(E)-heptenoic之庚酸鈣化合物的結晶多形體A、B、C、D、E、F,或其非晶型體,其中 |
|
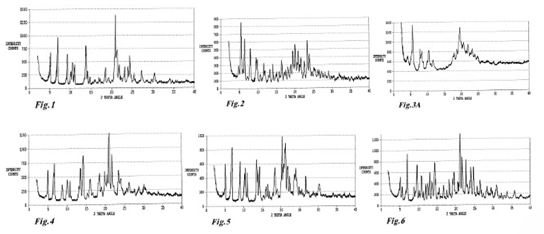
A) polymorph A exhibits a characteristic X-ray powder diffraction pattern (Fig.1) with characteristic peaks expressed in 2θ at 5.0 (s), 6.8 (s), 9.1 (s), 10.0 (w), 10.5 (m), 11.0 (m), 13.3 (vw), 13.7 (s), 14.0 (w), 14.7 (w), 15.9 (vw), 16.9 (w), 17.1 (vw), 18.4 (m), 19.1 (w), 20.8 (vs), 21.1 (m), 21.6 (m), 22.9 (m), 23.7 (m), 24.2 (s), 25.2 (w), 27.1 (m), 29.6 (vw), 30.2 (w), 34.0 (w);
A) 多形體A呈現一種X光粉末繞射分析之型態,其中峰形表現為2θ at 5.0 (s), 6.8 (s), 9.1 (s), 10.0 (w), 10.5 (m), 11.0 (m), 13.3 (vw), 13.7 (s), 14.0 (w), 14.7 (w), 15.9 (vw), 16.9 (w), 17.1 (vw), 18.4 (m), 19.1 (w), 20.8 (vs), 21.1 (m), 21.6 (m), 22.9 (m), 23.7 (m), 24.2 (s), 25.2 (w), 27.1 (m), 29.6 (vw), 30.2 (w), 34.0 (w)
|
B) polymorph B exhibits a characteristic X-ray powder diffraction pattern (Fig.2) with characteristic peaks expressed in 2θ at 4.6 (w), 5.3 (vs), 6.2 (s), 7.7 (s), 9.2 (m), 9.6 (m), 10.3 (w), 11.3 (m), 11.7 (w), 12.6 (vw), 13.0 (w), 13.9 (m), 14.7 (vw), 14.9 (w), 15.6 (w), 16.3 (m), 17.0 (vw), 17.4 (vw), 18.0 (w), 18.7 (m), 19.3 (m), 20.0 (s), 20.5 (w), 20.8 (m), 21.2 (w, shoulder), 21.5 (m), 22.4 (m), 23.2 (s), 23.8 (m), 24.4 (vw), 25.2 (w, broad), 26.0 (w), 26.4 (vw), 27.0 (w), 27.9 (vw), 28.9 (w);
B)多形體B呈現一種X光粉末繞射分析之型態,其中峰形表現為2θ at 4.6 (w), 5.3 (vs), 6.2 (s), 7.7 (s), 9.2 (m), 9.6 (m), 10.3 (w), 11.3 (m), 11.7 (w), 12.6 (vw), 13.0 (w), 13.9 (m), 14.7 (vw), 14.9 (w), 15.6 (w), 16.3 (m), 17.0 (vw), 17.4 (vw), 18.0 (w), 18.7 (m), 19.3 (m), 20.0 (s), 20.5 (w), 20.8 (m), 21.2 (w, shoulder), 21.5 (m), 22.4 (m), 23.2 (s), 23.8 (m), 24.4 (vw), 25.2 (w, broad), 26.0 (w), 26.4 (vw), 27.0 (w), 27.9 (vw), 28.9 (w) |
C) polymorph C exhibits a characteristic X-ray powder diffraction pattern (Fig.3A) with characteristic peaks expressed in 2θ at 4.1 (m), 5.6 (s), 7.8 (m), 8.3 (m), 10.3 (m), 11.6 (w), 17.5 (w), 17.9 (w),18.7 (m), 19.5 (s), 20.6 (m), 21.5 (vw), 21.9 (m), 23.1 (m), 24.0 (w), 24.8 (w);
C)多形體C呈現一種X光粉末繞射分析之型態,其中峰形表現為2θ at 4.1 (m), 5.6 (s), 7.8 (m), 8.3 (m), 10.3 (m), 11.6 (w), 17.5 (w), 17.9 (w),18.7 (m), 19.5 (s), 20.6 (m), 21.5 (vw), 21.9 (m), 23.1 (m), 24.0 (w), 24.8 (w) |
D) polymorph D exhibits a characteristic X-ray powder diffraction pattern (Fig.4) with characteristic peaks expressed in 2θ at 5.0 (m), 6.5 (m), 6.8 (s), 8.7 (m), 10.0 (m), 10.2 (m), 10.8 (m), 13.1 (w), 13.5 (m), 14.3 (s), 15.3 (vw), 16.1 (m), 16.8 (w), 18.2 (w), 18.5 (m), 19.0 (w), 19.9 (m), 20.5 (m), 21.0 (vs), 21.7 (s), 22.3 (w), 23.4 (m), 24.0 (m), 25.6 (w), 26.2 (m);
D)多形體D呈現一種X光粉末繞射分析之型態,其中峰形表現為2θ at 5.0 (m), 6.5 (m), 6.8 (s), 8.7 (m), 10.0 (m), 10.2 (m), 10.8 (m), 13.1 (w), 13.5 (m), 14.3 (s), 15.3 (vw), 16.1 (m), 16.8 (w), 18.2 (w), 18.5 (m), 19.0 (w), 19.9 (m), 20.5 (m), 21.0 (vs), 21.7 (s), 22.3 (w), 23.4 (m), 24.0 (m), 25.6 (w), 26.2 (m) |
E) polymorph E exhibits a characteristic X-ray powder diffraction pattern (Fig.5) with characteristic peaks expressed in 2θ at 4.4 (vw), 5.0 (s), 6.6 (s), 6.8 (s), 8.9 (s), 10.0 (m), 10.3 (s), 10.8 (m), 13.3 (s), 13.6 (m), 14.0 (s), 15.2 (vw), 15.9 (w), 16.4 (w), 16.9 (vw), 17.8 (vw), 18.3 (m), 18.9 (w), 20.2 (vs), 20.4 (m), 20.7 (m), 20.9 (m), 21.1 (vs), 21.6 (m), 21.7 (m), 22.3 (m), 23.5 (m), 23.8 (m), 24.1 (w), 24.7 (vw), 25.4 (vw), 26.6 (m), 30.2 (w), 34.0 (vw); and
E)多形體E呈現一種X光粉末繞射分析之型態,其中峰形表現為2θ at 4.4 (vw), 5.0 (s), 6.6 (s), 6.8 (s), 8.9 (s), 10.0 (m), 10.3 (s), 10.8 (m), 13.3 (s), 13.6 (m), 14.0 (s), 15.2 (vw), 15.9 (w), 16.4 (w), 16.9 (vw), 17.8 (vw), 18.3 (m), 18.9 (w), 20.2 (vs), 20.4 (m), 20.7 (m), 20.9 (m), 21.1 (vs), 21.6 (m), 21.7 (m), 22.3 (m), 23.5 (m), 23.8 (m), 24.1 (w), 24.7 (vw), 25.4 (vw), 26.6 (m), 30.2 (w), 34.0 (vw) |
F) polymorph F exhibits a characteristic X-ray powder diffraction pattern (Fig.6) with characteristic peaks expressed in 2θ at 5.1 (m), 5.6 (w), 7.0 (s), 8.8 (m), 9.6 (s), 10.2 (w), 10.9 (m), 11.3 (w), 11.9 (m), 12.5 (m), 13.0 (s), 13.7 (m), 14.4 (s), 14.7 (m), 15.3 (vw), 15.5 (w), 16.8 (m), 17.6 (w), 18.3 (m), 19.3 (m), 19.7 (m), 20.6 (m), 21.2 (vs), 21.8 (s), 22.8 (s), 23.1 (w), 23.8 (w, shoulder), 24.1 (s), 24.8 (s), 25.7 (m), 26.2 (vw), 26.6 (m), 26.9 (w), 28.4 (w), 29.5 (w), 29.8 (vw), 30.9 (m);
F)多形體F呈現一種X光粉末繞射分析之型態,其中峰形表現為2θ at 5.1 (m), 5.6 (w), 7.0 (s), 8.8 (m), 9.6 (s), 10.2 (w), 10.9 (m), 11.3 (w), 11.9 (m), 12.5 (m), 13.0 (s), 13.7 (m), 14.4 (s), 14.7 (m), 15.3 (vw), 15.5 (w), 16.8 (m), 17.6 (w), 18.3 (m), 19.3 (m), 19.7 (m), 20.6 (m), 21.2 (vs), 21.8 (s), 22.8 (s), 23.1 (w), 23.8 (w, shoulder), 24.1 (s), 24.8 (s), 25.7 (m), 26.2 (vw), 26.6 (m), 26.9 (w), 28.4 (w), 29.5 (w), 29.8 (vw), 30.9 (m) |
wherein, for each of said polymorphs, (vs) stands for very strong intensity; (s) stands for strong intensity; (m) stands for medium intensity; (w) stands for weak intensity; (vw) stands for very weak intensity.
在對前述個別多形體之描述中,(vs)代表非常強之繞射強度;(s)代表強繞射強度;(m)代表中等繞色強度;(w)代表弱繞射強度;(vw)代表非常弱之繞射強度 |
|
|