新流感疫情發燒,一周爆增15000例,世衛更預估全球可能20億人感染,專家認為遏阻蔓延最有效方式為施打疫苗。需求量應為人口之30%,因此產量為預防之瓶頸。流感疫苗類型有二:去活性與減毒性疫苗,製造方式分為傳統雞胚或細胞(含基因)工程。
傳統使用雞胚培養病毒係自1930年代即開始至今,確定培製疫苗的種株(master)病毒注入雞胚中,純化蛋白完成疫苗,最後通過無菌檢驗及人體試驗,完成疫苗製造(見圖一)。H1N1之流感種株如大陸:北京科興、華蘭生物等取自英國A型H1N1流感毒株(NYMCX179A);國光生技取自紐約NYMC種株等。
圖一、傳統方法
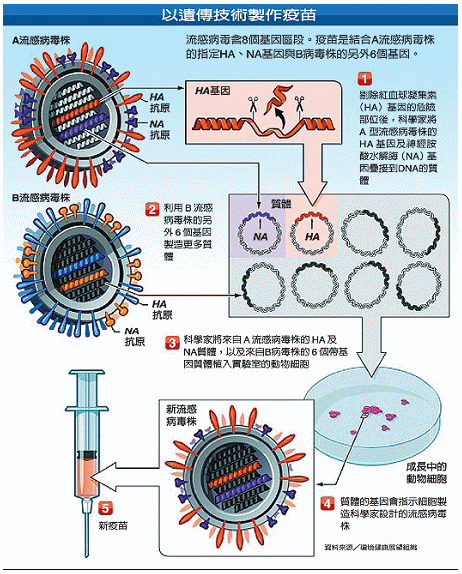
而且,以雞胚製造之疫苗並無法施打在對雞蛋過敏的人身上。目前,採用細胞(基因)工程製造新流感疫苗,即可避開過敏原問題。細胞(基因)工程製造,則分取流感病毒株HA及NA基因及另一流感病毒株其他6個基因質體,植入細胞培養。質體基因依照設定製造預期流感病毒株,經純化過程製成疫苗(圖二)。
細胞製造技術如荷蘭Crucell公司PER.C6細胞(人類視網膜細胞)培養及美國百特公司Vero細胞(非洲綠猴腎細胞)培養,特點在複製主成份活性物質能力佳,無須用動物培養,具高產量特質且可縮短製程降低製造成本。
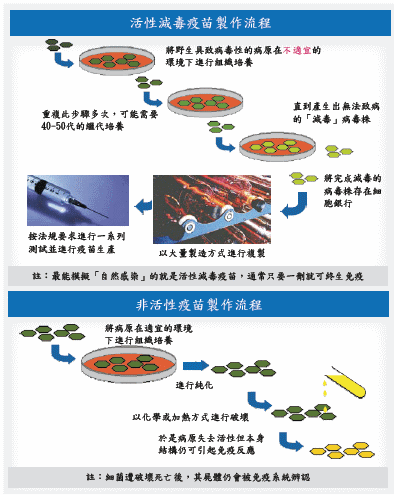
去活性疫苗是流感疫苗類型之一,主要將病毒顆粒固定後,透過被固定結構表面蛋白質來激活免疫力產生抗體,讓病毒無法繁殖。目前,以此種疫苗占絕大多數。另一種疫苗是減毒性疫苗,以改造病毒毒性或者透過改變溫度,使疫苗株對人體不具毒性,透過接種減毒疫苗在人體內繁殖,達到刺激免疫力效果(圖三)。兩者差異見表一:去活性與減毒性疫苗製程差異。
|
圖二、細胞(基因)工程方法
|
圖三、活性與非活性疫苗製造
|
 |
資料來源:2009年環境健康展望組織資料
|
| 表一、不同製造方法之疫苗特性比較 |
|
|
Inactivated flu vaccine Egg-based process
|
Inactivated flu vaccine Cell-culture process
|
Live attenuated Vaccine Egg-based process
|
Live attenuated vaccine Cell-culture process
|
|
Technology transfer and investment
|
|
|
|
|
|
Capital investment
|
High,>US$20m
|
Very high,
>US$100m
|
Low,US$2-3m
|
High
|
|
Time to establish manufacturing facility
|
3-4 years
|
4-5 years
|
1-2 years
|
>5 years
|
|
Technology requirement
|
High
|
Very high
|
Low
|
High
|
|
Manufacturing process
|
|
|
|
|
|
Cost per dose
|
Moderate
|
Moderate - high
|
Low
|
Moderate
|
|
Vaccine production time1
|
>8 weeks
|
>8 weeks
|
3-4 weeks
|
3-4 weeks
|
|
Pandemic vaccine lead time2
|
>12 weeks
|
>12 weeks
|
10 weeks
|
10 weeks
|
|
Dependence on external resources
|
High. Requires large numbers of eggs
|
Moderate. Requires large volumes of culture medium
|
High. Low numbers of eggs required, may need to be SPF
|
Low-moderate. Requires less volume of culture medium than IIV
|
|
Barriers to entry
|
|
|
|
|
|
Intellectual property
|
Low. No IP barrier on basic process
|
Potentially high. May need to license cell-line once it has approval
|
Potentially high.Will need to license CAIV
|
Potentially high. Will need to license cell line and CAIV
|
|
Regulatory pathway
|
Low. Standard method of production for flu vaccine
|
High. No cell lines are approved for flu vaccine production
|
Potentially high. Unlikely to meet current immunological correlates for protection
|
Potentially high. Unlikely to meet current correlates for protection. No cell line approved yet
|
|
Product
|
|
|
|
|
|
Immunogenicity
|
Good
|
Good
|
Potentially excellent. Should stimulate mucosal and cell-mediated responses
|
Potentially excellent. Should stimulate mucosal and cell-mediated responses
|
|
reactogenicity
|
Low
|
Very low
|
Potentially low due to intra-nasal delivery.
|
Potentially low due to intra-nasal delivery.
|
|

資料來源:2006年WHO資料
|
流感疫苗要有效與安全,綜合主要之步驟包括:
- (1)篩選病毒株。從病毒感染者樣本中篩選出適合生產疫苗的毒株,並確保它能完全代表流行病毒抗原特性,且具有較強生長能力。
- (2)疫苗研製。取得疫苗毒株後,進行大量病毒培養、收取病毒液、進行純化、鑑定等步驟製成病毒疫苗原液,加入定量佐劑[使用Al(OH)3]後分裝,製成臨床前評估疫苗。
- (3)臨床前評估。對疫苗進行理化生物學檢測,確定疫苗抗原含量等符合設計要求。
- (4)人體臨床試驗。由志願者接種疫苗後評估效價和安全性,確保疫苗對接種人群具有安全保護力。
經過嚴謹製造評估試驗,確保流感疫苗兼具安全與有效性,經衛生主管單位審查通過後就能量產上市。 以往所製造的流感疫苗為季節性流感疫苗,各廠皆依WHO建議選擇注射病毒之混合疫苗,但H1N1流感疫苗為今防疫之新產品,因此疫苗之安全、有效更為各界所重視。(1134字;圖3;表1)
--------------------------------------------------------------------------------------------------------------------------------------------